The GCC Biotech Transformation: Advancing Biotech R&D
Authored By: Nidhi Gupta, Principal Consultant, Healthcare & Lifesciences, Frost & Sullivan
The GCC region is actively shifting its focus from hydrocarbons by investing in R&D-anchored healthcare and biotechnology sectors. GCC countries have clear mentions in their national agendas such as Saudi Arabia's Vision 2030, UAE Vision 2031, Oman Vision 2040, and Qatar National Vision 2030 - each identifying life sciences and biotechnology as strategic pillars for economic diversification and sustainable development.
Within the broader life sciences landscape, biologics represent one of the most critical and transformative segments in modern pharmaceuticals. Unlike traditional small-molecule drugs, biologics—which include monoclonal antibodies, vaccines, cell therapies, gene therapies, and recombinant proteins—are derived from living organisms and offer highly targeted therapeutic interventions. This makes biologics particularly valuable in treating complex conditions.
The life sciences biotechnology sector in the GCC is particularly focused on biologics development and manufacturing. This focus is strategic for several reasons. First, biologics represent the fastest-growing segment within pharmaceuticals globally, driven primarily by advances in oncology and autoimmune disease treatments, two therapeutic areas experiencing exponential innovation. Second, biologics command higher value chains compared to traditional pharmaceuticals, offering significant economic returns. Third, as healthcare systems worldwide shift toward precision medicine, biologics provide the molecular specificity required for personalized treatment approaches.
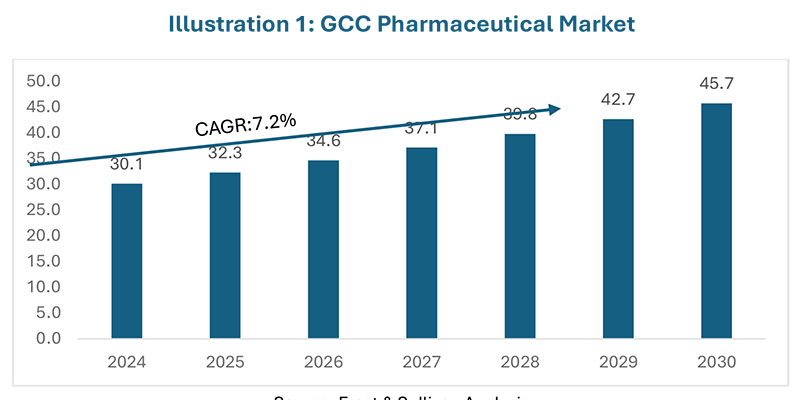
Pharmaceutical Market Growth Driven by Biologics
According to Frost & Sullivan analysis, the GCC pharmaceutical market was valued at approximately AED 110.5 billion (approximately USD 30.1 billion) in 2024, with projections indicating growth to AED 167.81 billion (approximately USD 45.7 billion) by 2030. This nearly fivefold expansion over next six years reflects not just increasing consumption but a fundamental transformation in therapeutic capabilities across the region. A significant driver of this transformation is the rapidly expanding biologics segment, which represents one of the highest-growth categories within the broader pharmaceutical market.
What makes biologics particularly compelling as a growth segment is the therapeutic focus driving this expansion. Oncology biologics—including checkpoint inhibitors, CAR-T cell therapies, and targeted monoclonal antibodies—are revolutionizing cancer treatment paradigms. As cancer incidence rises globally and regionally, the demand for these precision oncology treatments continues to accelerate. Similarly, autoimmune disease biologics such as TNF inhibitors, IL-17 inhibitors, and JAK inhibitors have transformed the management of conditions like rheumatoid arthritis, psoriasis, and inflammatory bowel diseases, creating sustained demand growth.
The region's epidemiological profile amplifies this opportunity. With rising incidences of cancer (expected to rise sharply to around 103,000 cases by 2040 from 42,475 new cancer cases in 2020) and autoimmune conditions alongside existing high prevalence of metabolic diseases (with obesity prevalence quite high among GCC countries: Kuwait (38%), Qatar (35%), Saudi Arabia (35%), and the United Arab Emirates (32%))), the region represents a growing market for advanced biologics.1 More importantly, this creates a compelling rationale for developing indigenous capabilities rather than remaining solely dependent on imports.
Starting with the Basics: Manufacturing as Foundation
The GCC began with manufacturing. Over the past decade, countries-built fill-finish facilities and distribution networks. Saudi Arabia partnered with AstraZeneca in 2020 for COVID-19 vaccine production through the Saudi Vaccine and Biopharmaceutical Corporation. The UAE's Julphar worked with Sandoz on biosimilars, while Hayat Pharmaceuticals in Saudi Arabia expanded its biologics production facilities.
This wasn't the final goal—it was training. Manufacturing teaches quality standards, regulatory requirements, and supply chain complexities. That hands-on knowledge creates the foundation for better research decisions later. The King Abdullah University of Science and Technology (KAUST) Bioscience Research Center and the UAE's Abu Dhabi Stem Cells Center demonstrate how regional institutions-built R&D capacity alongside manufacturing expertise. At KAUST, the Bioscience Research Center and the Computational Bioscience Research Center (CBRC) have established strong capabilities in bioinformatics, artificial intelligence–enabled biotechnology, stem cell research, and infectious diseases. These programs provide a robust scientific foundation for biologics innovation, effectively linking advanced computational research with experimental and translational biosciences.
Building on this research-led base, the Abu Dhabi Stem Cells Center (ADSCC) serves as a critical bridge between discovery and application. ADSCC has achieved a regional first by developing clinical grade induced pluripotent stem cells (iPSCs) under Good Manufacturing Practice (GMP) protocols. This milestone strengthens the Middle East’s position in regenerative medicine and cell-based therapies, while also reinforcing the manufacturing standards required for biologics and advanced therapy products.
Beyond these anchor institutions, the establishment of Qatar Biobank and the development of Dubai Science Park further illustrate the region’s systematic approach to building a comprehensive biologics ecosystem. Qatar Biobank plays a foundational role as a centralized repository of biological samples and health data, supporting large-scale genetic research and translational studies essential for biologics and precision medicine development. Complementing this, the Dubai Biotechnology and Research Park (DuBiotech) provides an integrated environment that brings together research laboratories, manufacturing facilities, and incubators within a dedicated free-zone framework, supporting the progression from research to product development and commercialization.
Why R&D Is Now a Priority in the GCC?
The GCC is shifting strongly toward R&D because its populations have unique genetic profiles that are understudied globally.
By studying regional genetics, GCC countries can develop treatments for their own populations and generate insights useful globally. Research on rare diseases often helps unlock therapies for common diseases like cancer, diabetes, and cardiovascular conditions.
This shift is backed by serious capital. The UAE committed AED 300 billion (approx USD 81.7 million) to pharma and biotech over five years, while Saudi Arabia targets SAR 130 billion (USD 34.6 billion) GDP contribution from biotech by 2040. These investments are funding facilities, talent, and partnerships.
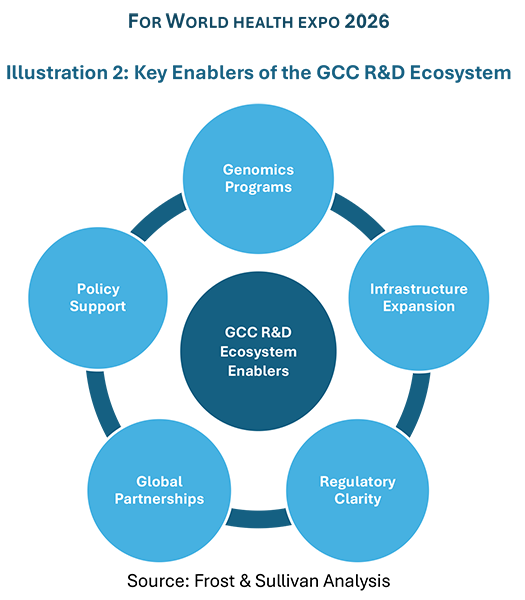
1. Genomics Programs
Large national genome initiatives are underway across the GCC. The UAE aims to sequence one million genomes, Saudi Arabia has collected over 63,000 samples, and Qatar has sequenced more than 20,000 genomes. These programs directly support personalized medicine and drug discovery.
Biobanks link genetic data with health records, enabling research in cancer, cardiovascular disease, and newborn screening. This data actively guides therapeutic targeting, not just storage.
2. Infrastructure Expansion
The region has built advanced research infrastructure. G42 operates the largest omics facility in the region. Dubai Science Park and Dammam Valley offer incentives, shared labs, and incubation support. These are integrated ecosystems covering research, manufacturing, and commercialization.
Biobanks connect samples with long-term clinical data, helping researchers understand treatment response differences and speeding translation to clinical use.
3. Regulatory Clarity
Saudi Arabia’s SFDA biosimilar guidelines align with global standards, reducing approval risk. The UAE’s federal genome law protects data privacy while enabling research. Regulatory alignment across GCC countries further improves market access and scale.
4. Global Partnerships
The GCC is partnering with global leaders to accelerate learning. M42 works with AbbVie and AstraZeneca on genomics and cancer research. BridgeBio has partnered with Burjeel on rare diseases. Saudi Arabia has collaborated with Illumina, while Qatar has partnered with institutions such as Mayo Clinic and Johns Hopkins. These partnerships combine global expertise with regional data and patients.
5. Policy Support
UAE has allowed 100% foreign ownership in biotech, attracting global companies.
What Comes Next: An Export-Oriented, IP-Rich Biopharma Future
The investments made so far in the GCC—new research centers, manufacturing plants, partnerships with global pharma companies, and national genomics programs—are already in place. Building on this foundation, the GCC aims to become a source of biologics, biosimilars, and cell therapies for global markets, shifting its image of primarily being a buyer of advanced therapies.
1. Strong Fiscal Capacity Backing Long-Cycle Biologics Development
The GCC’s biologics ambition is supported by demonstrable fiscal strength. Sovereign wealth funds across the region manage trillions of dollars in long-term capital, enabling sustained funding for sectors that require multi-year investment. Saudi Arabia’s Public Investment Fund and Abu Dhabi’s government-backed investment entities have committed multi-billion-dollar capital allocations to healthcare, life sciences, genomics programs, and pharmaceutical manufacturing under national diversification strategies. This financial depth will allow governments to fund biologics R&D, advanced therapy manufacturing, and clinical research over five to ten-year timelines, which many emerging biotech hubs cannot consistently sustain.
2. Shift Toward IP-Rich Startups and Platform-Based Innovation
Rather than focusing only on contract manufacturing or research services, the GCC is increasingly targeting ownership of intellectual property. Public funding programs and partnerships are encouraging the development of platform technologies—such as biologics manufacturing processes, cell and gene therapy delivery systems, and diagnostics platforms—that can be applied across multiple products. This marks a deliberate move toward scalable, asset-driven biotech companies rather than service-only models.
3. A Maturing Ecosystem Enabling R&D Scale-Up
The GCC’s biologics ecosystem now extends beyond individual facilities. Incubators and accelerators provide startups with mentorship, funding access, and industry connections. GMP-ready manufacturing facilities allow early-stage companies to move into clinical and commercial production without heavy upfront capital investment, and several such facilities are already operational across Saudi Arabia and the UAE. Clinical trial capacity is expanding through coordinated networks, such as the UAE Clinical Trials Network and Saudi Arabia’s National Center for Clinical Trials, established in 2023. In parallel, national genomics programs and digitized health records are being integrated with AI-powered analytics, enabling faster target identification, smarter trial design, and more efficient regulatory submissions.
Looking Ahead
The GCC has built the foundation for a globally competitive biotechnology sector. Investments in genomics, infrastructure, partnerships, and liberal regulations are translating into real capabilities. Rare disease research leverages unique regional genetic data. Clinical trials meet international standards. Manufacturing facilities are expanding into advanced modalities.
The region's approach combines strategic advantages—geography, capital, digital infrastructure—with deliberate capability building in R&D, regulation, and clinical development. As these elements mature, the GCC will contribute meaningful innovations to global biopharma while addressing its own healthcare needs. The transition from ambition to execution is underway, and the progress suggests a sector that will be both regionally significant and globally relevant.





